Dr. Nickolas Charistos
Chemist
Nickolas Charistos Chemist PhD
Assistant Professor
Laboratory of Quantum and Computational Chemistry - School of Chemistry - AUTh
Office 402, 2nd floor, Old Chemistry Building
2310 997801
nicharis@chem.auth.gr
Research
Publications
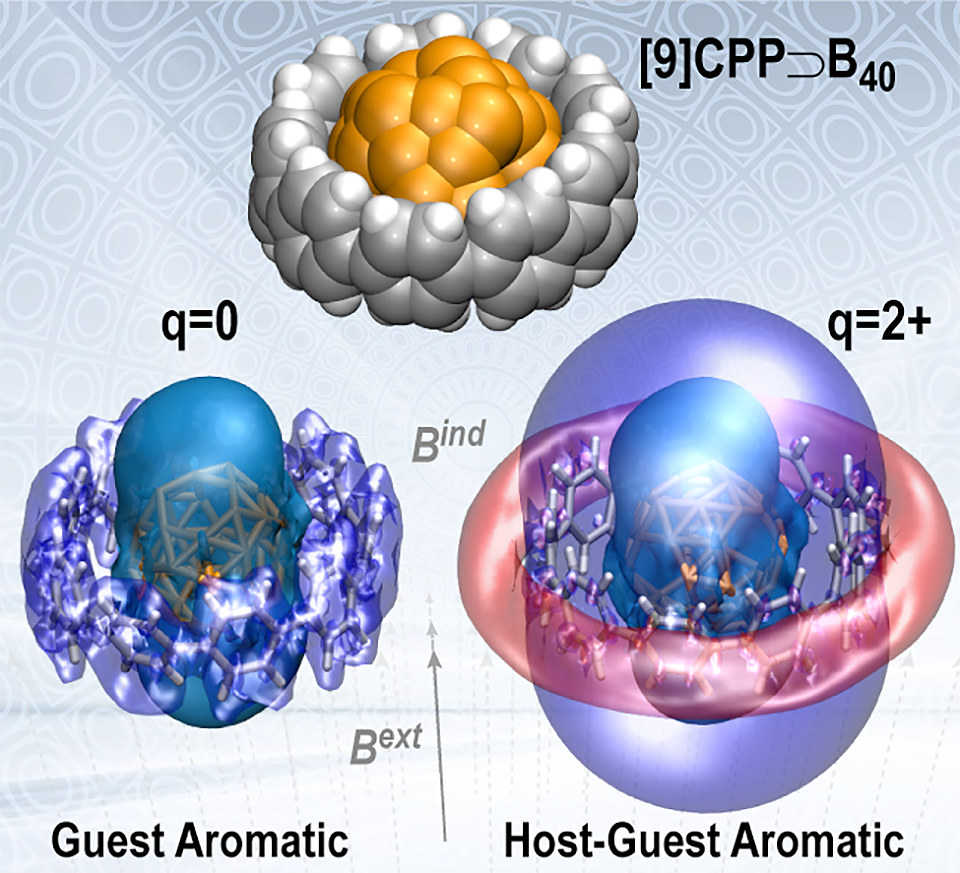
Lingas R., , Muñoz-Castro* A.
BOROSPHERENE IN THE NANOHOOP:
Complexation and Aromaticity of Neutral and Dioxidized Cycloparaphenylene Supramolecules with B40 And C60 Fullerenes
Chemistry - A European Journal 2024, e202402027 , DOI: 10.1002/chem.202402027
Lingas R., , Muñoz-Castro* A.
Charge Delocalization and Aromaticity of Doubly Reduced Double-walled Carbon Nanohoops
Physical Chemistry Chemical Physics 2023, 25 , 19481-19491, DOI: 10.1039/D3CP01994B 2023 PCCP HOT Article

Lingas R., , Muñoz-Castro* A.
Local and Global Aromaticity under Rotation. Analysis of Two- and Three-Dimensional Representative Carbon Nanostructures
Physical Chemistry Chemical Physics 2023, 25 , 14285-14293, DOI: 10.1039/D3CP00569K
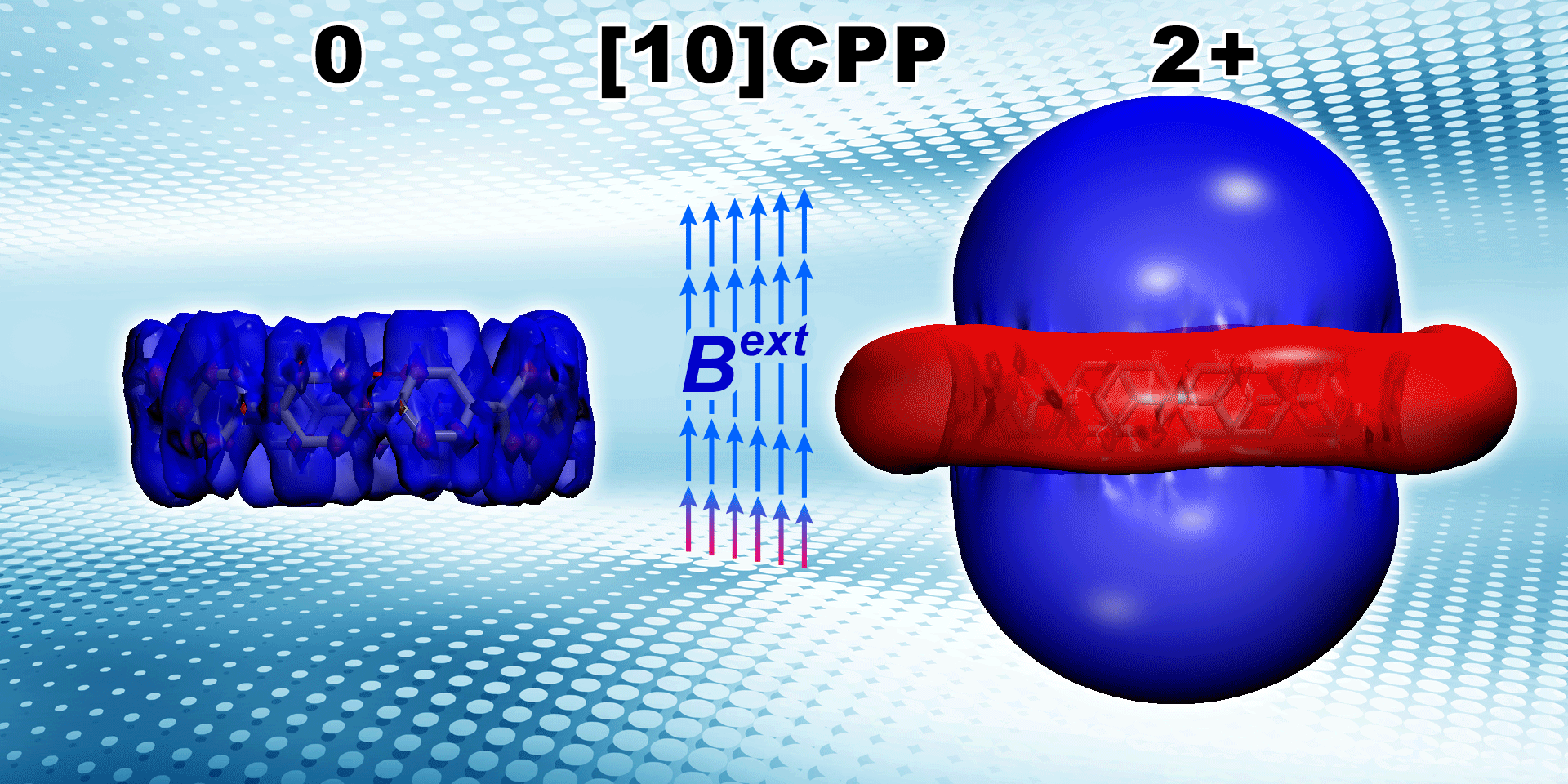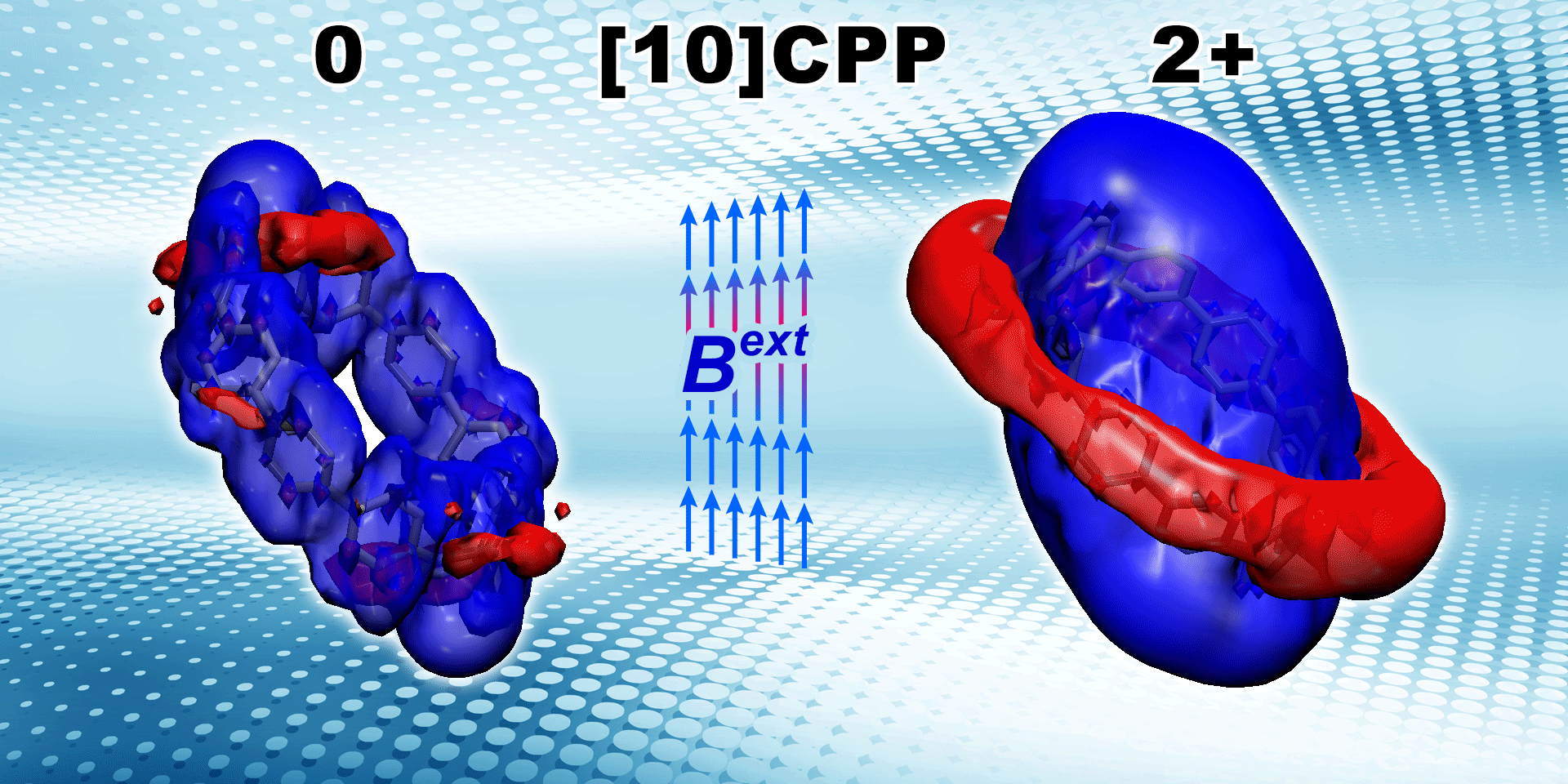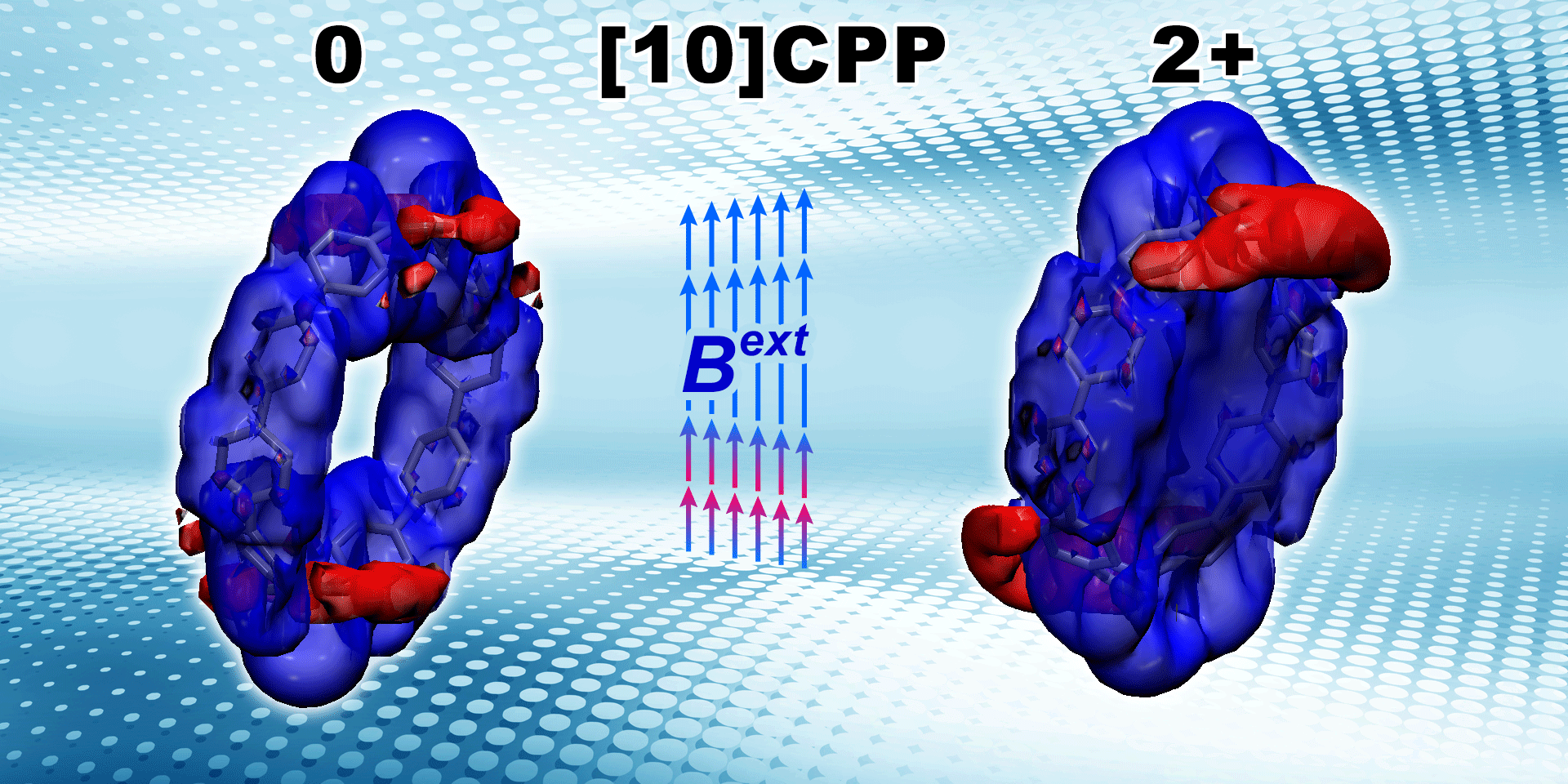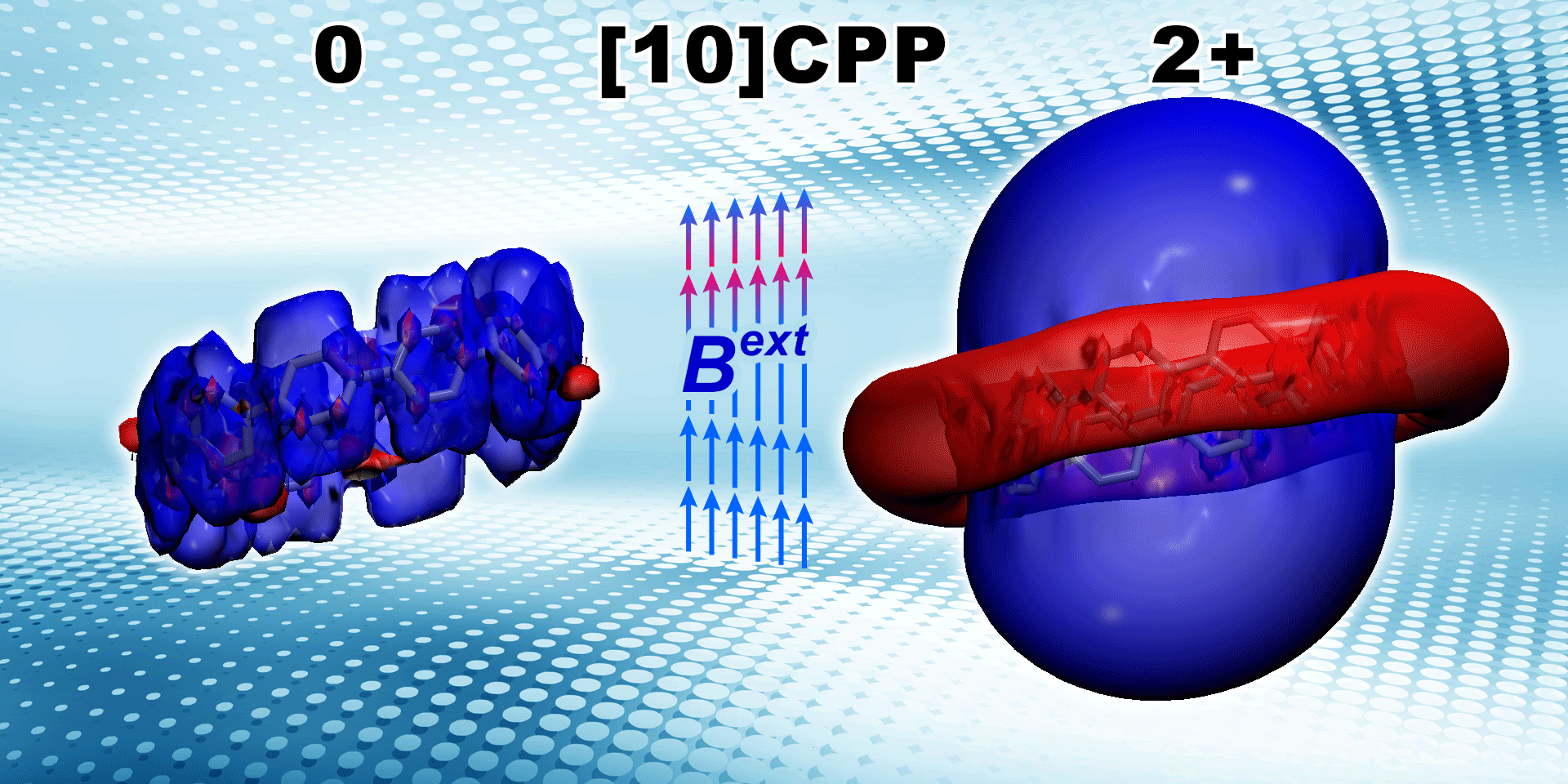
Lingas R., , Muñoz-Castro* A.
Aromaticity of ortho and meta 8-cycloparaphenylene and their dications: Induced magnetic field analysis with localized and delocalized orbitals in strained nanohoops
ChemPhysChem 2021, 22 (8) , 741-751 , DOI: 10.1002/cphc.202100057
, Muñoz-Castro* A.
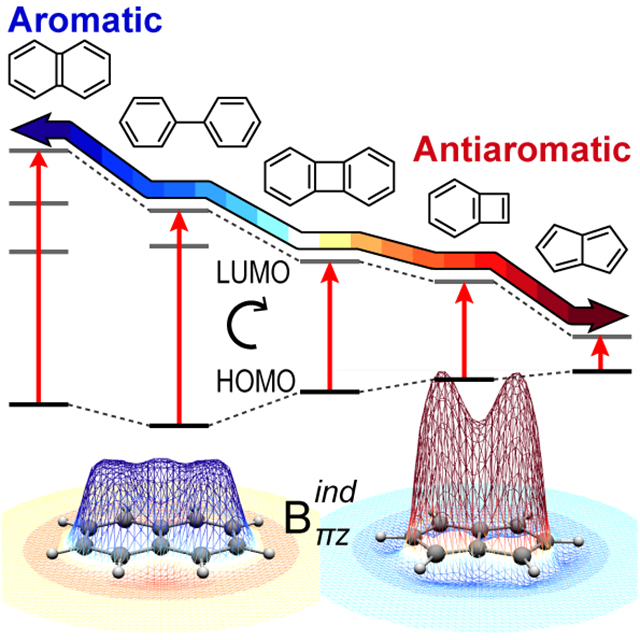
Induced Magnetic Field in sp-Hybridized Carbon Rings: Analysis of Double Aromaticity and Antiaromaticity in Cyclo[2n]carbon Allotropes
Physical Chemistry Chemical Physics 2020, 22 , 9240-9249 , DOI: 10.1039/D0CP01252A 2020 PCCP HOT Article
, Muñoz-Castro* A.
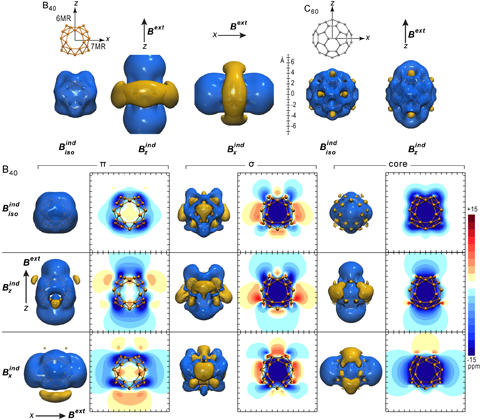
Double aromaticity of B40 Fullerene: Induced magnetic field analysis of π and σ delocalization in boron cavernous structure
Physical Chemistry Chemical Physics 2019, 21 , 20232-20238 DOI: 10.1039/C9CP04223G
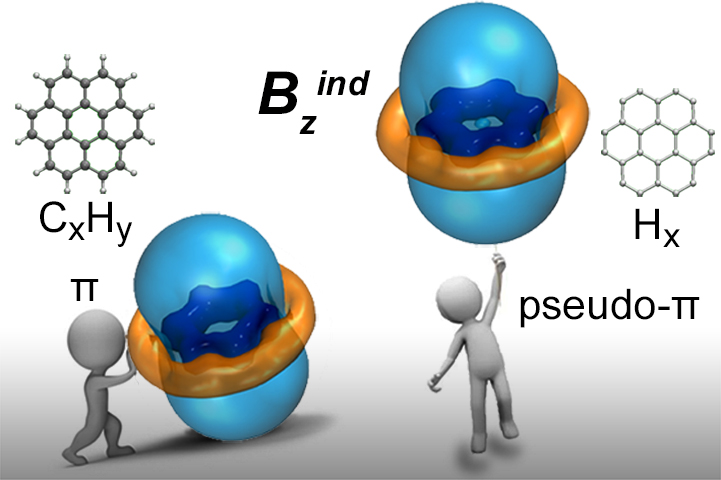
, Muñoz-Castro* A. and Sigalas M. P.
The pseudo-π model of the Induced Magnetic Field: Fast and Accurate Visualization of Shielding and Deshielding Cones in Planar Conjugated Hydrocarbons and Spherical Fullerenes
Physical Chemistry Chemical Physics 2019, 21, 6150-6159 DOI: 10.1039/C9CP00836E
Supplementary Material: Interactive Explorable Visualizations
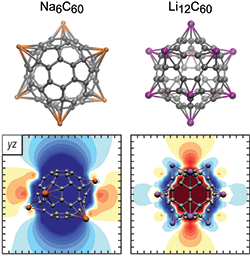
and Muñoz-Castro* A.
Τhe Induced Magnetic Field of Fullerenes. Role of σ- and π- contributions to Spherical Aromatic, Non-Aromatic and Antiaromatic Character in C60q (q = +10, 0, -6, -12), and Related Alkali-Metal Decorated Building Blocks, Li12C60 and Na6C60
Journal of Physical Chemistry C 2018, 122 (17) , 9688-9698 DOI: 10.1021/acs.jpcc.8b02419
, Papadopoulos A., Nikopoulos T, Muñoz-Castro A. and Sigalas* M.
Canonical Orbital Contributions to the Magnetic Fields Induced by Global and Local Diatropic and Paratropic Ring Currents
Journal of Computational Chemistry 2017, 38 (30) , 2594-2604 DOI: 10.1002/jcc.24917
Papadopoulos A. G., and Muñoz-Castro* A.
Magnetic Response of Aromatic Rings Under Rotation: Aromatic Shielding Cone of Benzene Upon Different Orientations of the Magnetic Field
ChemPhysChem 2017, 18 (12) , 1499–1502 DOI: 10.1002/cphc.201700279
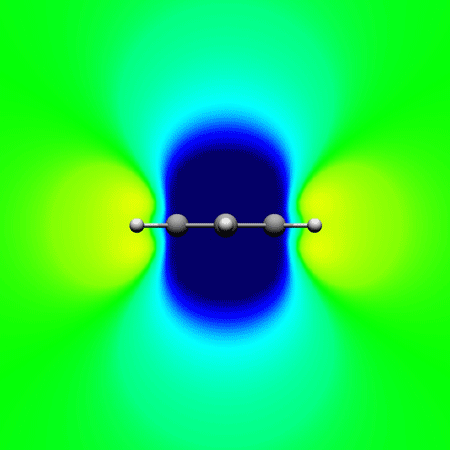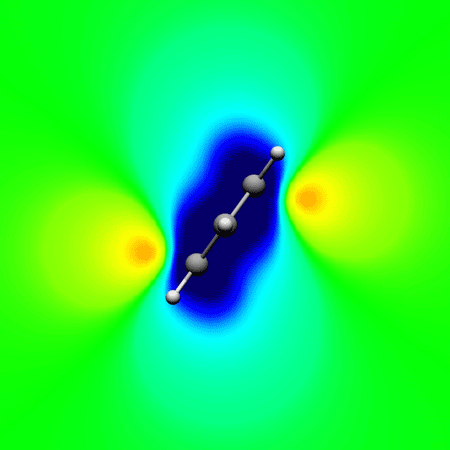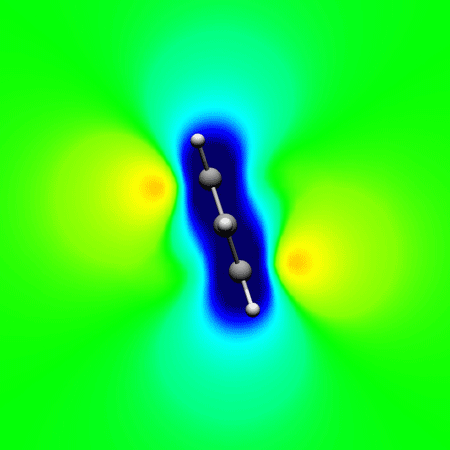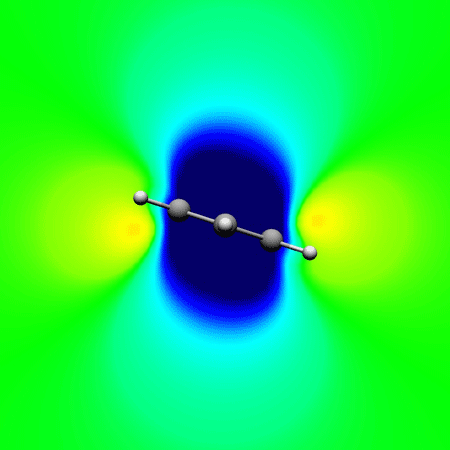
, Papadopoulos A. G. and Sigalas* M.
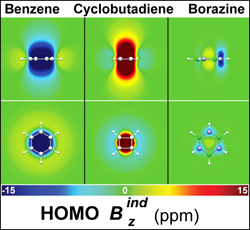
Interpretation of electron delocalization in benzene, cyclobutadiene, and borazine based on visualization of individual molecular orbital contributions to the induced magnetic field
Journal of Physical Chemistry A 2014 , 118 (6) , 1113–22 DOI: 10.1021/jp411410r
Papers
- R. Lingas, N. D. Charistos* and A. Muñoz-Castro*
BOROSPHERENE IN THE NANOHOOP: Complexation and Aromaticity of Neutral and Dioxidized Cycloparaphenylene Supramolecules with B40 And C60 Fullerenes
Chemistry - A European Journal 2023, e202402027, DOI: 10.1002/chem.202402027 - R. Lingas, N. D. Charistos* and A. Muñoz-Castro*
Charge Delocalization and Aromaticity of Doubly Reduced Double-walled Carbon Nanohoops
Physical Chemistry Chemical Physics 2023, 25, 19481-19491, DOI: 10.1039/D3CP01994B 2023 PCCP HOT Article - R. Lingas, N. D. Charistos* and A. Muñoz-Castro*
Local and Global Aromaticity under Rotation. Analysis of Two- and Three-Dimensional Representative Carbon Nanostructures
Physical Chemistry Chemical Physics 2023, 25, 14285-14293, DOI: 10.1039/D3CP00569K - Mesías Orozco-Ic,* Nickolas D. Charistos, Alvaro Muñoz-Castro, Rafael
Islas, Dage Sundholm,* and Gabriel Merino*
Core-electron contributions to the molecular magnetic response
Physical Chemistry Chemical Physics 2022, 24, 12158-12166, DOI: 10.1039/D1CP05713H - R. Lingas, N. D. Charistos* and A. Muñoz-Castro*
Aromaticity of ortho and meta 8-cycloparaphenylene and their dications: Induced magnetic field analysis with localized and delocalized orbitals in strained nanohoops
ChemPhysChem 2021, 22(8), 741-751, DOI: 10.1002/cphc.202100057 - P. L. Rodríguez-Kessler, N. D. Charistos, R. B. King* and A. Muñoz-Castro*
On the Formation of Spherical Aromatic Endohedral Buckminsterfullerene. Evaluation of M@C60 (M=Cr, Mo, W) From Relativistic DFT Calculations
Physical Chemistry Chemical Physics 2020, 22, 14268-14275, DOI: 10.1039/D0CP02475A - N. D. Charistos* and A. Muñoz-Castro*
Induced Magnetic Field in sp-Hybridized Carbon Rings: Analysis of Double Aromaticity and Antiaromaticity in Cyclo[2n]carbon Allotropes
Physical Chemistry Chemical Physics 2020, 22, 9240-9249, DOI: 10.1039/D0CP01252A 2020 PCCP HOT Article - M. Orozco-Ic, J. Barroso, N. D. Charistos, A. Muñoz-Castro* and G. Merino*
Consequences of the curvature on the induced magnetic field: Case of helicenes
Chemistry—A European Journal 2020, 26 (1), 326-330, DOI: 10.1002/chem.201904390 Very Important Paper - Charistos* N. D., Muñoz-Castro* A.
Double aromaticity of B40 Fullerene: Induced magnetic field analysis of π and σ delocalization in boron cavernous structure
Physical Chemistry Chemical Physics 2019, 21 (36), 20232-20238, DOI: 10.1039/C9CP04223G - Karamanis* P., Charistos N. D., Sigalas M. P. and Rérat M.
Polyaromatic Systems Combining Increasing Optical Gaps and Amplified Nonlinear Optical Properties. A Comprehensive Theoretical Study on B3N3 Doped Nanographenes
The Journal of Physical Chemistry C 2019, 123 (34), 21135-21149, DOI: 10.1021/acs.jpcc.9b05543 - Charistos* N. D., Muñoz-Castro* A. and Sigalas M.
P.
The pseudo-π model of the Induced Magnetic Field: Fast and Accurate Visualization of Shielding and Deshielding Cones in Planar Conjugated Hydrocarbons and Spherical Fullerenes
Physical Chemistry Chemical Physics 2019, 21, 6150-6159 DOI: 1039/C9CP00836E - Charistos N. D., Jin P. and Muñoz-Castro* A.
Aromatic Character of Oh-C24N24. A Cavernous Nitride Fullerene Bearing N4-Macrocycle Motifs
International Journal of Quantum Chemistry 2019 DOI: 1002/qua.25919 - Ortolan O. A., Charistos N. D, Guajardo-Maturana R., Olea C., Caramori G.
F., Parreira R. and Muñoz-Castro A.
On the Cation-π Capabilities of Small all sp2-Carbon Host Structures. Evaluation of [6.8]3Cyclacene from Relativistic DFT Calculations
International Journal of Quantum Chemistry 2019, 119(4), e25811 DOI: 1002/qua.25811 - Charistos* N. D. and Muñoz-Castro* A.
The Induced Magnetic Field of Fullerenes. Role of σ- and π- contributions to Spherical Aromatic, Non-Aromatic and Antiaromatic Character in C60q (q = +10, 0, -6, -12), and Related Alkali-Metal Decorated Building Blocks, Li12C60 and Na6C60.
Journal of Physical Chemistry C 2018, 122 (17), 9688-9698 DOI: 1021/acs.jpcc.8b02419 - Charistos* N. D., Papadopoulos A., Nikopoulos T,
Muñoz-Castro A. and Sigalas* M.
Canonical Orbital Contributions to the Magnetic Fields Induced by Global and Local Diatropic and Paratropic Ring Currents.
Journal of Computational Chemistry 2017, 38 (30), 2594–2604 DOI: 1002/jcc.24917 - Papadopoulos A. G, Charistos* N. D. and Muñoz-Castro* A.
Magnetic Response of Aromatic Rings Under Rotation: Aromatic Shielding Cone of Benzene Upon Different Orientations of the Magnetic Field.
ChemPhysChem 2017, 18 (12), 1499–1502 DOI: 1002/cphc.201700279 - Papadopoulos A. G., Charistos* N. D.. and
Muñoz-Castro* A.
On the Role of Heteroatoms in Aromatic Rings. Insights from 10π Main Group Elements Heterorings [(EH)2S2N4]q (E = C, P, B, Si, Al and q = 0, −2).
New Journal of Chemistry 2016, 40 (6), 5090–5098 DOI: 10.1039/C5NJ03573B - A. G. Papadopoulos, N. D.
Charistos, K. Kyriakidou and M.P. Sigalas*
Study of Electron Delocalization in 1,2-, 1,3-, and 1,4-Azaborines Based on the Canonical Molecular Orbital Contributions to the Induced Magnetic Field and Polyelectron Population Analysis.
Journal of Physical Chemistry A 2015, 119(39), 10091–10100 DOI: 1021/acs.jpca.5b06027 - N. D.
Charistos*, A. G. Papadopoulos and M. P. Sigalas*
Interpretation of electron delocalization in benzene, cyclobutadiene, and borazine based on visualization of individual molecular orbital contributions to the induced magnetic field.
Journal of Physical Chemistry A 2014, 118(6), 1113–22 DOI: 1021/jp411410r - Antonoglou L. D., Kostelidou T. N., Charistos N. D., Sigalas* M. P.
Investigating Chemistry Students’ Skills to Mentally Manipulate (Rotation & Reflection) 2D Symbolic Molecular Representations.
Procedia - Social and Behavioral Sciences 2014, 152, 517–522 DOI: 1016/j.sbspro.2014.09.208 - Koutalas V. G., Antonoglou L. D., Charistos N. D., Sigalas* M. P.
Investigation of Students’ Ability to Transform and Translate 2D Molecular Diagrammatic Representations and Its Relationship to Spatial Ability and Prior Chemistry Knowledge.
Procedia - Social and Behavioral Sciences 2014, 152, 698–703 DOI: 1016/j.sbspro.2014.09.265 - Papadopoulos A. G., Charistos N. D., Sigalas* M. P.
Aromaticity Variation in BN Substituted Triphenylene: A Theoretical Study.
AIP Conference Proceedings 2012, 1504, 1223–1226 DOI: 1063/1.4772148 - L.D. Antonoglou, N.D.
Charistos and M.P. Sigalas*
Design, development and implementation of a technology enhanced hybrid course on molecular symmetry: Students' outcomes and attitudes.
Chemistry Education Research and Practice 2011, 12(4), 454-468 DOI: 1039/C0RP90013C - D. Antonoglou, N.D. Charistos, M.P. Sigalas*
Design of Molecular Visualization Educational Software for Chemistry Learning.
In T.B. Scott and J. I. Livingston (Eds) Leading-Edge Educational Technology, 55 – 81, Nova Puplishers, New York, 2008 - A. Tsipis*, C. E. Kefalidis and N. D. Charistos.
Ligand-Effects on the Stability, Conformational Preference and Diatrophic Response of 3- Membered Hydrocopper Rings.
In T. W. Cartere and K. S. Verley (Eds), Coordination Chemistry Research Progress, 201 – 215, Nova Puplishers, New York, 2008 - A. Tsipis* and N. D. Charistos
How the Aromatic 4-Membered Hydrido-Bridged Copper Rings Respond to Successive Nucleophilic Attack?
The Open Mechanical Engineering Journal 2008, 2, 12 DOI: 10.2174/1874155X00802010012 - N. D. Charistos, C.A. Tsipis and M. P. Sigalas*
3D Molecular Symmetry Shockwave: A Web Application for Interactive Visualization and Three-Dimensional Perception of Molecular Symmetry.
Journal of Chemical Education 2005, 82, 1741 DOI: 10.1021/ed082p1741.2 - N. D. Charistos, C.A. Tsipis and M. P. Sigalas*
3DNormalModes Shockwave. Three Dimensional Perception of Molecular Normal Modes on the Web.
Journal of Chemical Education 2004, 81, 1231 DOI: 10.1021/ed081p1231.2 - N. D. Charistos, V.I. Teberekidis, C.A. Tsipis and M.P. Sigalas*
Design and Development of a Multimedia Educational Tool for Interactive Visualization and Three Dimensional Perception of Vibrational Spectra Data of Molecules.
Education and Information Technologies 2003, 8, 369 DOI: 10.1023/B:EAIT.0000008677.08249.98 - P. Sigalas*, N. D. Charistos, V.I. Teberekidis and C. A. Tsipis
3DNormalModes 1.0.
Journal of Chemical Education 2003, 80, 1222 DOI: 10.1021/ed080p1222.2
International Conferences
- A. G. Papadopoulos, N. D. Charistos, M. P. Sigalas.
Visualization of molecular orbitals contributions to the induced magnetic field of heterocyclic isocoronene analogues.
25th Conference on Current Trends in Computational Chemistry, November 2017, Jackson, MS, United States - A. G. Papadopoulos, N. D. Charistos, A. Arvanitidis, M. P. Sigalas.
Visualization of the canonical molecular orbital contributions to the induced magnetic field of B‐Trisubstituted Borazines.
15th International Congress of Quantum Chemistry, June 2015, Beijing, China - N. D. Charistos, A. G. Papadopoulos, T. A. Nikopoulos, M. P. Sigalas.
Visualization of Differences and Similarities of the Magnetic Response of π and σ- Molecular Orbitals in Seven-Membered Boron Wheels.
10th Congress of the World Association of Theoretical and Computational Chemists, WATOC, October 2014, Santiago, Chile - A. G. Papadopoulos, N. D. Charistos, A. G. Arvanitidis, M. P. Sigalas.
Substituents' Effects on π Electron Delocalization in B-Trisubstituted Borazines Based on Visualization of Molecular Orbitals' Contributions to the Induced Magnetic Field.
10th Congress of the World Association of Theoretical and Computational Chemists, WATOC, October 2014, Santiago, Chile - N. D. Charistos, Maria Palassopoulou, and Michael P. Sigalas.
Teaching Structural Biochemistry Using Jmol Based Interactive Molecular Visualization Material and Protein Data Bank.
12th European Conference on Research in Chemistry Education, ECRICE, July 2014, Jyväskylä, Finland - G. Koutalas, L. D. Antonoglou, N. D. Charistos, M. P. Sigalas.
How students translate and transform molecular diagrammatic representations? A study based on matching items questionnaires, eye movements’ patterns and verbal explanations.
12th European Conference on Research in Chemistry Education, ECRICE, July 2014, Jyväskylä, Finland - P. Sigalas, N. D. Charistos, V. Koutalas, M. Palassopoulou and L.D.
Antonoglou.
Molecular Visualization in Chemistry Education. From symmetry principles to platonic solids, transition metal complexes and proteins’ structure.
First African Conference on Research In Chemistry Education [ACRICE-1], 2013 Adis Ababa, Ethiopia - D. Antonoglou, N. D. Charistos, M. P. Sigalas.
Exploring Criteria for Selecting Proper Orientations of 2D and 3D Molecular Representations in Chemistry Education.
11th European Conference on Research in Chemistry Education, ECRICE, July 2012, Rome, Italy - A. G. Papadopoulos, N. D. Charistos, M. Sigalas.
Molecular Orbital Contributions to the Induced Magnetic Field οf Benzene, Cyclobutadiene, Borazine, 1,2-, 1,3- and 1,4-Azaborines.
9th Triennial Congress of the World Association of Theoretical and Computational Chemists, WATOC, July 2011, Sandiago de Compostela, Spain - N. D. Charistos, L. D. Antonoglou, V. Koutalas and M. P. Sigalas.
Design of Molecular Visualization Educational Software for Learning and Teaching Molecular Symmetry: From Learning Objects to Online Course Material.
10th European Conference on Research in Chemistry Education, ECRICE, July 2010, Krakow, Poland - D. Antonoglou, N.D. Charistos, M.P. Sigalas.
Design, Development, Implementaton and Assessment of a Hybrid Course on Molecular Symmetry — A pilot project.
The 4th International Conference Research in Didactics of the Sciences, DidSci, July 2010, Krakow, Poland - L. Antonoglou, V. Koutalas, M. Palassopoulou, N. D. Charistos, M. Sigalas.
Molecular Visualization in Chemistry Education. From symmetry principles to platonic solids, transition metal complexes and proteins' structure.
Gordon Research Conference on Visualization in Science and Education, GRC, July 2009, Magdalen College, Oxford, UK - P. Sigalas and N. D. Charistos.
3DMolSym - Chemistry Classic Award Winner.
MERLOT International Conference, August 2008, Minneapolis USA - N. D. Charistos and M. P. Sigalas.
Standalone and Web Applications for Interactive Visualization and Three Dimensional Perception of Molecular Symmetry Elements and Normal Modes.
Gordon Research Conference on Visualization in Science and Education, GRC, July 2005, Queen's College, Oxford, UK - N. D. Charistos and M. P. Sigalas.
Standalone and Web Applications for Interactive Visualization and Three Dimensional Perception of Molecular Symmetry Elements and Normal Modes.
International Conference on Computational Methods in Sciences and Engineering, ICCMSE, October 2005, Lutraki, Greece - N. D. Charistos, V.I. Teberekidis, C.A. Tsipis, M.P. Sigalas.
3DNormalModes: A program for Three Dimensional Perception of Normal Modes.
6th World Congress of Theoretically Oriented Chemists, WATOC, 2002, Lugano, Switzerland - N. D. Charistos, V.I. Teberekidis, C.A. Tsipis, M.P. Sigalas.
Development of Educational Programs for Visualization and Three Dimensional Perception of Symmetry Elements and Normal Modes of Vibration.
4th European Conference on Computational Chemistry, EUCO-CC4, 2002, Assisi, Italy
National (Greek) Conferences
- Δ. Γανίτη, Λ. Πασσιάς, Μ.-Π. Βλαχολιά, Β. Κουταλάς, Ν. Χαριστός, Μ. Σιγάλας
Αξιολόγηση Ευχρηστίας και Αποτελεσματικότητας Γνωστικού Εργαλείου Σχεδίασης και Χειρισμού Συντακτικών Τύπων Οργανικών Ενώσεων
11ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, Φλώρινα, 2019 - Β. Κουταλάς, Π. Κατικαρίδου, Ν. Χαριστός, Μ. Σιγάλας
Εφαρμογή Τεχνικών Οφθαλμικών Καταγραφών για τη Μελέτη Χειρισμού και Επεξεργασίας της Πληροφορίας από Χρήστες Πολυαναπαραστασιακού Εκπαιδευτικού Περιβάλλοντος με Αντικείμενο τις Προβολές Fischer & Newman
10ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, Ρέθυμνο, 2017 - Ν. Χαριστός, Β. Κουταλάς, Μ. Βλαχολιά, Κ. Σάλτα, Χ. Τζουγκράκη, Μ. Σιγάλας
2DrawChemQuiz: Σχεδιασμός, ανάπτυξη και εφαρμογή ενός γνωστικού εργαλείου εξάσκησης στη σχεδίαση και χειρισμό συντακτικών τύπων
10ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, Ρέθυμνο, 2017 - Α. Βουκλής, Ε. Παρισοπούλου, Ν. Χαριστός, Α. Γιαννακουδάκης, Π. Γιαννακουδάκης
Η Χρησιμοποίηση των Δυνατοτήτων του CMS Joomla στο Σχεδιασμό και Υλοποίηση Ιστότοπου με Θέμα την Ηλεκτροχημεία.
8o Πανελλήνιο Συνέδριο των Εκπαιδευτικών για τις ΤΠΕ, 26‐28 Ιουνίου, Σύρος, 2015. - Β. Γ. Κουταλάς, Ν. Δ. Χαριστός, Μ. Π. Σιγάλας.
Σχεδιασμός και Ανάπτυξη Εκπαιδευτικού Ιστότοπου με Δυναμικά Διασυνδεόμενες Μοριακές Αναπαραστάσεις για την Κατανόηση των Προβολών Fischer και Newman.
8o Πανελλήνιο Συνέδριο των Εκπαιδευτικών για τις ΤΠΕ, 26‐28 Ιουνίου, Σύρος, 2015. - Β. Γ. Κουταλάς, Λ. Δ. Αντώνογλου, Ν. Δ. Χαριστός, Μ. Π. Σιγάλας.
Βαθμός Κατανόησης και Ευχέρεια Χειρισμού Δισδιάστατων Διαγραμματικών Μοριακών Αναπαραστάσεων και Ανάπτυξη Εκπαιδευτικού Λογισμικού για τη Βελτίωση τους.
12o Συνέδριο Χημείας Ελλάδος Κύπρου, Θεσσαλονίκη, 2015 - Α. Γ. Παπαδόπουλος, Ν. Δ. Χαριστός, Α. Αρβανιτίδης, Μ. Π. Σιγάλας.
Οπτικοποίηση και ανάλυση της επίδρασης της υποκατάστασης στον π- ηλεκτρονιακό απεντοπισμό σε Β-τριυποκατεστημένες βοραζίνες.
12o Συνέδριο Χημείας Ελλάδος Κύπρου, Θεσσαλονίκη, 2015 - Ν. Δ. Χαριστός, Α. Γ. Παπαδόπουλος, Θ. Νικόπουλος, Μ. Π. Σιγάλας.
Οπτικοποίηση και ανάλυση της μαγνητικής απόκρισης των π- και σ- μοριακών τροχιακών επταμελών μοριακών τροχών βορίου.
12o Συνέδριο Χημείας Ελλάδος Κύπρου, Θεσσαλονίκη, 2015 - Β. Γ. Κουταλάς, Λ. ∆. Αντώνογλου, Ν. ∆. Χαριστός, Μ. Π. Σιγάλας.
∆ιερεύνηση των δυσκολιών που αντιµετωπίζουν οι φοιτητές στην κατανόηση και το νοητικό χειρισµό δισδιάστατων διαγραµµατικών µοριακών αναπαραστάσεων
9ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστηµών και Νέων Τεχνολογιών στην Εκπαίδευση, Θεσσαλονίκη, 2015 - Α. Βουκλής, Ε. Παρισοπούλου, Ν. Χαριστός, Α. Γιαννακουδάκης, Π. Γιαννακουδάκης.
∆ιερεύνηση των Απόψεων των Φοιτητών Σχετικά µε τη Μελέτη και την Κατανόηση Της Ηλεκτροχηµείας µέσω Χρήσης Εκπαιδευτικού Ιστοτόπου και Κατάλληλα Σχεδιασµένων Ερωτήσεων Αυτοαξιολόγησης.
9ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστηµών και Νέων Τεχνολογιών στην Εκπαίδευση, Θεσσαλονίκη, 2015 - Β. Γ. Κουταλάς, Ν. ∆. Χαριστός, Μ. Π. Σιγάλας.
Σχεδιασµός και Ανάπτυξη Εκπαιδευτικού Λογισµικού Χειρισµού Τρισδιάστατων Μοριακών Μοντέλων και ∆ισδιάστατων ∆ιαγραµµατικών Αναπαραστάσεων µε Αντικείµενο τις Προβολές Fischer και Newman.
9ο Πανελλήνιο Συνέδριο στη Διδακτική των Φυσικών Επιστηµών και Νέων Τεχνολογιών στην Εκπαίδευση, Θεσσαλονίκη, 2015 - Λ. Αντώνογλου, , Θ. Κωστελίδου, Ν. Χαριστός, Μ. Σιγάλας.
Διερεύνηση Οπτικοχωρικής Ικανότητας Φοιτητών Χημείας. Εργαλεία Εκτίμησης Δεξιοτήτων Νοητικής Οπτικοποίησης και Χειρισμού της Τρισδιάστατης Μοριακής Δομής.
8ο Πανελλήνιο Συνέδριο Διδακτικής των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, Βόλος, 2013 - B. Κουταλάς, N. Γιωργάς, N. Χαριστός, M. Σιγάλας.
Επανασχεδιασμός του εκπαιδευτικού λογισμικού για το μάθημα της Χημείας Γ΄ Γυμνάσιου «Ο Θαυμαστός Κόσμος της Χημείας» με την αξιοποίηση των δυνατοτήτων ενός σύγχρονου συστήματος διαχείρισης περιεχομένου.
10o Συνέδριο Ε.Ε.Ε.Π. - Δ.Τ.Π.Ε - Η Εκπαίδευση στην Εποχή των ΤΠΕ, Αθήνα, 2013 - Λ.Δ. Αντώνογλου, Ν.Δ. Χαριστός, Μ.Π. Σιγάλας.
Διερεύνηση της δεξιότητας της νοητικής μεταφοράς ανάμεσα σε Tρισδιάστατες & Δισδιάστατες αναπαραστάσεις της τετραεδρικής μοριακής δομής από φοιτητές Χημείας.
7ο Πανελλήνιο Συνέδριο Διδακτικής των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση (ΚΟΔΙΦΕΕΤ), Αλεξανδρούπολη, 2011 - Ν. Δ. Χαριστός, Α.Γ. Παπαδόπουλος, Θ. Νικόπουλος, M.Π. Σιγάλας.
Οπτικοποίηση της Συνεισφοράς των Μοριακών Τροχιακών στο Επαγόμενο Μαγνητικό Πεδίο Επίπεδων Κυκλικών Μορίων.
21ο Πανελλήνιο Συνέδριο Χημείας, Θεματική Ενότητα: Φυσική και Θεωρητική Χημεία, Θεσσαλονίκη, 2011 - Λ. Αντώνογλου, Ν. Χαριστός, Μ. Σιγάλας.
Διερεύνηση της ικανότητας φοιτητών χημείας στο νοητικό χειρισμό και τη μετάφραση 2D και 3D μοριακών οπτικοποιήσεων.
21ο Πανελλήνιο Συνέδριο Χημείας, Θεματική Ενότητα: Χημεία και Εκπαίδευση, Θεσσαλονίκη, 2011 - Λ.Δ. Αντώνογλου, Ν.Δ. Χαριστός, Μ.Π. Σιγάλας.
Σχεδιασμός, ανάπτυξη, εφαρμογή και αξιολόγηση της αποτελεσματικότητας του υβριδικού μοντέλου διδασκαλίας της μοριακής συμμετρίας.
7ο Πανελλήνιο Συνέδριο με Διεθνή Συμμετοχή, Οι ΤΠΕ στην Εκπαίδευση - Ελληνική Επιστημονική Ένωση Τεχνολογιών Πληροφορίας & Επικοινωνιών στην Εκπαίδευση (ΕΤΠΕ), Κόρινθος, 2010 - Λ.Δ. Αντώνογλου, Ν.Δ. Χαριστός, Μ.Π. Σιγάλας.
Σχεδιασμός & Ανάπτυξη Υβριδικού Μοντέλου Διδασκαλίας της Μοριακής Συμμετρίας με Χρήση Τρισδιάστατης Μοριακής Οπτικοποίησης.
6ο Πανελλήνιο Συνέδριο Διδακτικής των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, (ΚΟΔΙΦΕΕΤ), Φλώρινα, 2009 - Μ. Παλασοπούλου, Ν. Χαριστός, Μ. Σιγάλας
Τρισδιάστατη Οπτικοποίηση των Πρωτεϊνών ως Εκπαιδευτικό Εργαλείο σε Περιβάλλον WWW.
6ο Πανελλήνιο Συνέδριο Διδακτικής των Φυσικών Επιστημών και Νέων Τεχνολογιών στην Εκπαίδευση, (ΚΟΔΙΦΕΕΤ), Φλώρινα, 2009 - Μ. Σιγάλας, Α. Γιαννακουδάκης, Χ. Τσουγκράκη, Β. Αγγελόπουλος, Α. Τζαμτζής, Ν. Χαριστός και
Λ. Δ. Αντώνογλου. Υποστηρικτικό Εκπαιδευτικό Λογισμικό για το Μάθημα της Χημείας Γυμνασίου.
5ο Πανελλήνιο Συνέδριο Ε.Τ.Π.Ε., “Oι Τεχνολογίες της Πληροφορίας και των Επικοινωνιών στην Εκπαίδευση”, Θεσσαλονίκη, 2006 - Ν. Δ. Χαριστός και Μ. Π. Σιγάλας.
Ανάπτυξη Εκπαιδευτικού Λογισμικού Μοριακής Μοντελοποίησης και Εφαρμογή του σε Θέματα Συμμετρίας και Κανονικών Τρόπων Δόνησης των Μορίων.
5ο Πανελλήνιο Συνέδριο Ε.Τ.Π.Ε., “Oι Τεχνολογίες της Πληροφορίας και των Επικοινωνιών στην Εκπαίδευση”, Θεσσαλονίκη, 2006 - Ν. Δ. Χαριστός, Κ.Α. Τσίπης, Μ. Π. Σιγάλας.
Χρήση Τεχνολογιών Τρισδιάστατων Γραφικών για τη Μοντελοποίηση και Τρισδιάστατη Απεικόνιση των Κανονικών Τρόπων Δόνησης και της Συµµετρίας των Μορίων. 2ο Πανελλήνιο Συνέδριο “Πληροφορική και Εκπαίδευση”, Θεσσαλονίκη, 2004
Software Development
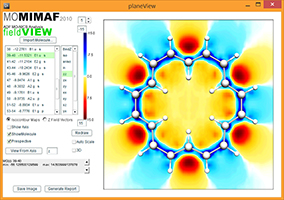
MIMAF
Computational Chemistry Tool
ADF output processing and molecular visualization of contour maps and vector maps of Molecular Induced Magnetic Fields
2008-2019
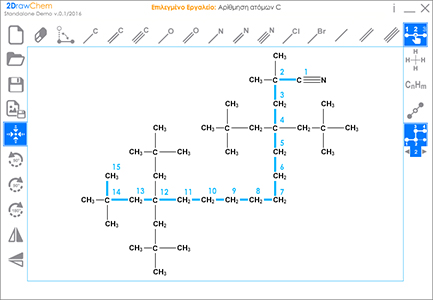
2DrawChem
Educational Chemistry Software and Research Tool
Chemical structural formulas modelling and drawing software. Development of interactive structural formulas chemistry problems
2013-2015

MIE (Matching Items Environment)
Educational Chemistry Research Tool
Interactive Digital Instruments for the assesment of visuospatial thinking in chemsitry problem solving
2013
Molix3D
Educational Chemistry Software
Software package for the molecular visualization of polymers, isomers, enantiomers, peptides and functional groups
2007
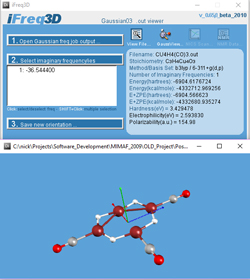
iFreq3D
Computational Chemistry Tool
Gaussian output processing and molecular visualization of NICS-scan diagrams
2006
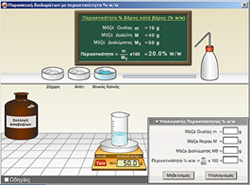
juniorLab
Educational Chemistry Software
Chemistry Laboratory Simulation
2005
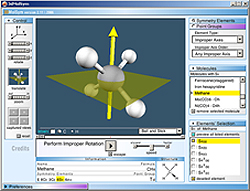
3DMolSym
Educational Chemistry Software
Molecular visualization of symmetry elements and symmetry operations
2004
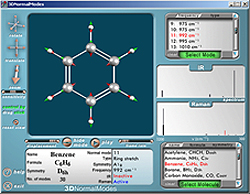
3DNormalModes
Educational Chemistry Software
Molecular visualization of normal modes of vibrations
2003
Web Design and Development
Computational Chemistry Video Tutorials (in Greek)
Lab 1 - Z-Matrix & GaussView
Lab 2 - Μοριακή Μηχανική
Lab 3 - Σύνολα Βάσης
Lab 4 - Βελτιστοποίηση Γεωμετρίας - Σάρωση PES
Lab 5 - Μοριακές Ιδιότητες - Οπτικοποίηση
Lab 6 - Κανονικοί Τρόποι Δόνησης
Teaching 2021-22
- K104 Structuring, Presentation and Transmission of Chemical Information
- K107 Development of Multimedia Material and e-Learning in Chemistry and Chemistry Teaching
- H10 Computational Chemistry
- K101 Molecular Modeling
- ΜΧ116 Applications of ICT in Teaching Chemistry
- Μ5Υ1/1 Techniques and Tools for the Visualization of Molecular Structure, Chemical Properties and Data
External Collaborators
- Prof. Alvaro Muñoz-Castro / Universidad Autónoma de Chile
Testzone - Fun Stuff
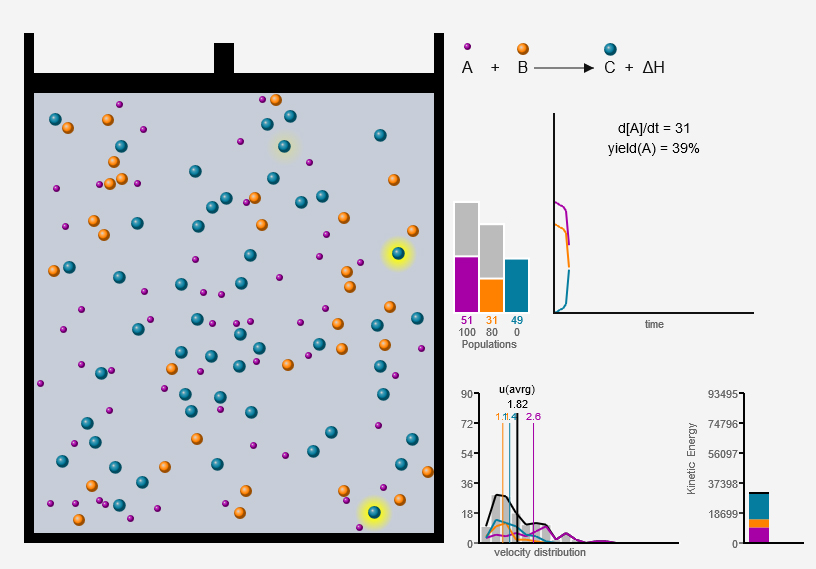
Chemical Reaction Simulator (working example)
Canvas/Javascript/HTML5
Chemical reaction simulation based on particle elastic collision.
2019 (work in progress)
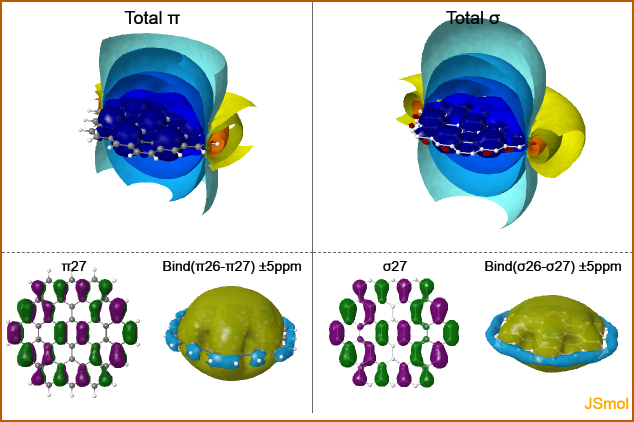
Pseudo-Pi Model
JSmol webpage
Interactive Explorable Visualizations of Induced Magnetic Field Isosurfaces with JSmol
2018
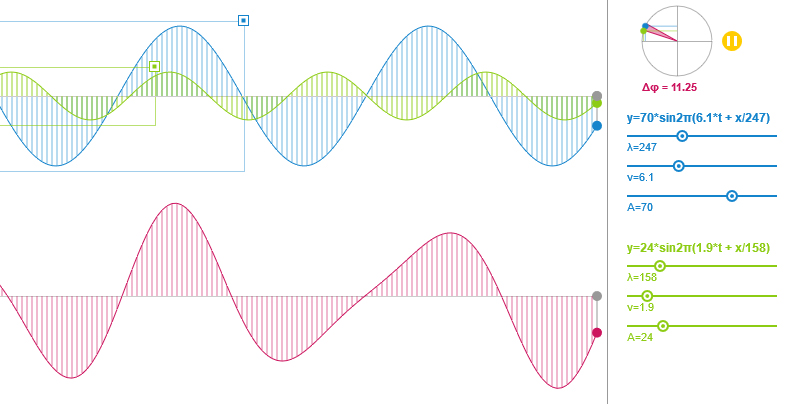
Wave Superposition Simulation
Canvas/Javascript/HTML5
Manipulating simple sliders the user can adjust the wavelength, the frequency and the amplitude of two animated sine waves, visualize the resulting sum and explore related scientific concepts.
2011
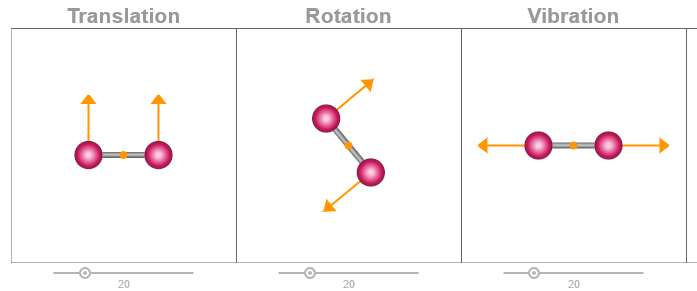
Diatomic Motion Simulation
Canvas/Javascript/HTML5
A simple interactive animation of translational, rotational and vibrational motion of a diatomic molecule.
2011
Research Grants
-
Design and development of online educational chemistry software
Student's scientific literacy development in formal and informal education with the use of virtual laboratories.
Principal Investigator
2018-2021
ID 97155